Vitamin C, Titrating to Tolerance----------------------------------- ----- Robert F. Cathcart,M.D. ----- --- Allergy, Environmental, and --- ----- Orthomolecular Medicine ----- ------- Orthopedic Medicine ------- --- 127 Second Street, Suite 4 --- --- Los Altos, California, USA --- -------- Fax:650-949-5083 --------- -----------------------------------
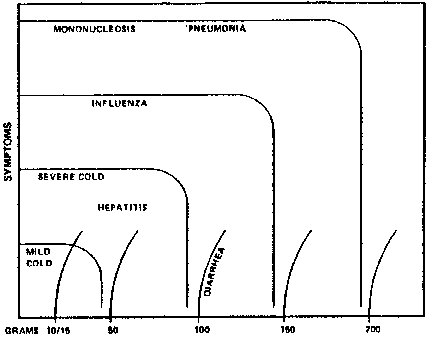
Medical Hypotheses, 7:1359-1376, 1981.VITAMIN C, TITRATING TO BOWEL TOLERANCE, ANASCORBEMIA, AND ACUTE INDUCED SCURVYRobert F. Cathcart, III, M.D. Allergy, Environmental, and Orthomolecular Medicine 127 Second Street, Los Altos, California 94022, USA Telephone 650-949-2822 ABSTRACTA method of utilizing vitamin C in amounts just short of the doses which produce diarrhea is described (TITRATING TO BOWEL TOLERANCE). The amount of oral ascorbic acid tolerated by a patient without producing diarrhea increases somewhat proportionately to the stress or toxicity of his disease. Bowel tolerance doses of ascorbic acid ameliorate the acute symptoms of many diseases. Lesser doses often have little effect on acute symptoms but assist the body in handling the stress of disease and may reduce the morbidity of the disease. However, if doses of ascorbate are not provided to satisfy this potential draw on the nutrient, first local tissues involved in the disease, then the blood, and then the body in general become deplete of ascorbate (ANASCORBEMIA and ACUTE INDUCED SCURVY). The patient is thereby put at risk for complications of metabolic processes known to be dependent upon ascorbate. INTRODUCTIONOver the past ten-year period I have treated over 9,000 patients with large doses of vitamin C (Cathcart 1, 2, 3, 4, 5). The effects of this substance when used in adequate amounts markedly alters the course of many diseases. Stressful conditions of any kind greatly increase utilization of vitamin C. Ascorbate excreted in the urine drops markedly with stresses of any magnitude unless vitamin C is provided in large amounts. However, a more convenient and clinically useful measure of ascorbate need and presumably utilization is the BOWEL TOLERANCE. The amount of ascorbic acid which can be taken orally without causing diarrhea when a person is ill sometimes is over ten times the amount he would tolerate if well. This increased bowel tolerance phenomenon serves not only to indicate the amount which should be taken but indicates the unsuspected and astonishing magnitude of the potential use that the body has for ascorbate under stressful conditions. If this massive draw on the small ascorbate stores of the body is not fully satisfied, the condition of ANASCORBEMIA results. The deficit of ascorbate probably starts in the tissues directly involved in the disease and then spreads to other tissues of the body. A condition of localized and then systemic acute scurvy is produced. This ACUTE INDUCED SCURVY leads to poor healing and ultimately to complications involving other systems of the body. Much of the original work with large amounts of vitamin C was done by Fred R. Klenner, M.D. (6, 7, 8, 9) of Reidsville, North Carolina. Klenner found that viral diseases could be cured by intravenous sodium ascorbate in amounts up to 200 grams per 24 hours. Irwin Stone (10, 11, 12) pointed out the potential of vitamin C in the treatment of many diseases, the inability of humans to synthesize ascorbate, and the resultant condition hypoascorbemia. Linus Pauling (13, 14) reviewed the literature on vitamin C and has led the crusade to make known its medical uses to the public and the medical profession. Ewan Cameron in association with Pauling (15, 16, 17) has shown the usefulness of ascorbate in the treatment of cancer. BOWEL TOLERANCE METHODIn 1970, I discovered that the sicker a patient was, the more ascorbic acid he would tolerate by mouth before diarrhea was produced. At least 80% of adult patients will tolerate 10 to 15 grams of ascorbic acid fine crystals in 1/2 cup water divided into 4 doses per 24 hours without having diarrhea. The astonishing finding was that all patients, tolerant of ascorbic acid, can take greater amounts of the substance orally without having diarrhea when ill or under stress. This increased tolerance is somewhat proportional to the toxicity of the disease being treated. Tolerance is increased some by stress (e.g., anxiety, exercise, heat, cold, etc.)(see FIGURE I). Admittedly, increasing the frequency of doses increases tolerance perhaps to half again as much, but the tolerances of sometimes over 200 grams per 24 hours were totally unexpected. Representative doses taken by tolerant patients titrating their ascorbic acid intake between the relief of most symptoms and the production of diarrhea were as follows: TABLE I - USUAL BOWEL TOLERANCE DOSES
GRAMS ASCORBIC ACID NUMBER OF DOSES
CONDITION PER 24 HOURS PER 24 HOURS
normal 4 - 15 4 - 6
mild cold 30 - 60 6 - 10
severe cold 60 - 100+ 8 - 15
influenza 100 - 150 8 - 20
ECHO, coxsackievirus 100 - 150 8 - 20
mononucleosis 150 - 200+ 12 - 25
viral pneumonia 100 - 200+ 12 - 25
hay fever, asthma 15 - 50 4 - 8
environmental and
food allergy 0.5 - 50 4 - 8
burn, injury, surgery 25 - 150+ 6 - 20
anxiety, exercise and
other mild stresses 15 - 25 4 - 6
cancer 15 - 100 4 - 15
ankylosing spondylitis 15 - 100 4 - 15
Reiter's syndrome 15 - 60 4 - 10
acute anterior uveitis 30 - 100 4 - 15
rheumatoid arthritis 15 - 100 4 - 15
bacterial infections 30 - 200+ 10 - 25
infectious hepatitis 30 - 100 6 - 15
candidiasis 15 - 200+ 6 - 25
FIGURE 1. REPRESENTATIVE
DOSES TO TREAT ACUTE SYMPTOMS OF
1) Note that disease symptom curves indicate very little effect on acute symptoms until doses of 80-90% of bowel tolerance are reached. Perhaps it is only near tolerance doses that the ascorbate is pushed into the primary sites of the disease. 2) Suppression of symptoms in some instances may not be total; but usually it is very significant and often the amelioration is complete and rapid. 3) Hepatitis may require 30 to 100 grams. TITRATING TO BOWEL TOLERANCEThe maximum relief of symptoms which can be expected with oral doses of ascorbic acid is obtained at a point just short of the amount which produces diarrhea. The amount and the timing of the doses are usually sensed by the patient. The physician should not try to regulate exactly the amount and timing of these doses because the optimally effective dose will often change from dose to dose. Patients are instructed on the general principles of determining doses and given estimates of the reasonable starting amounts and timing of these doses. I have named this process of the patient determining the optimum dose, TITRATING TO BOWEL TOLERANCE. The patient tries to TITRATE between that amount which begins to make him feel better and that amount which almost but not quite causes diarrhea. I think it is only that excess amount of ascorbate not absorbed into the body which causes diarrhea; what does not reach the rectum, does not cause diarrhea. It is interesting to know, when one speculates on the exact cause of this diarrhea, that while a hypertonic solution of sodium ascorbate is being administered intravenously, the amount of ascorbic acid tolerated orally actually increases. THE 100 GRAM COLDWhen a person is ill the amount of ascorbic acid he can ingest without diarrhea being produced increases somewhat proportionally to the severity or the toxicity of the disease. A cold severe enough to permit a person to take 100 grams of ascorbic acid per 24 hours during the peak of the disease, I call a 100 GRAM COLD. INDIVIDUAL RESPONSESPerhaps one of the most important principles in ORTHOMOLECULAR MEDICINE is BIOCHEMICAL INDIVIDUALITY (18). Every individual responds to substances differently. Vitamin C is no exception. However, at least 80% of my patients tolerated ascorbic acid well. Admittedly, there were relatively few older patients in my practice. Infants, small children, and teenagers tolerate ascorbic acid well and can take, proportionate to their body weight, larger amounts than adults. Older adults tolerate lesser amounts and have a higher percentage of nuisance difficulties. Patients with multiple food intolerances may have more difficulties but should attempt taking ascorbate because of benefits often obtained. For several years while I was treating only sick people with ascorbic acid, I was unaware of the number of people who had nuisance problems with maintenance doses. The tolerance of the sick person to ascorbate is so high as to prevent many of the complaints one would have if he were well. When ascorbic acid is prescribed to a sick person, the beneficial effect is obvious enough so that few complain of the gas and diarrhea. With illness the effects of an overdose do not last long because of the rapid rate of utilization. It is important for the physician to understand the principles of treating this vast majority of tolerant persons. Patients frequently underdose themselves and need professional guidance to push the doses to effective levels. The small number of persons, especially elderly persons, intolerant to oral doses are in my experience able to take intravenous ascorbate without difficulties. Additionally, patients with severe problems may need to be treated intravenously if very high doses will have to be maintained for some time for adequate suppression of symptoms. ANASCORBEMIA -- ACUTE INDUCED SCURVYIt is well established that certain symptoms are associated with an almost total lack of vitamin C within the body. Symptoms of scurvy include lassitude, malaise, bleeding gums, loss of teeth, nosebleeds, bruising, hemorrhages in any part of the body, easy infections, poor healing of wounds, deterioration of joints, brittle and painful bones, and death, etc. It is thought that this disease only occurs with dietary deprivation of vitamin C. However, an analogous condition is produced as follows: Well-nourished humans usually contain not much more than 5 grams of vitamin C in their bodies. Unfortunately, the majority of people have far less ascorbate than this amount in their bodies and are at risk for many problems related to failure of metabolic processes dependent upon ascorbate. This condition is called CHRONIC SUBCLINICAL SCURVY (12). If a disease is toxic enough to allow for the person's potential consumption of 100 grams of vitamin C, imagine what that disease must be doing to that possible 5 grams of ascorbate stored in the body. A condition of ACUTE INDUCED SCURVY is rapidly induced. Some of this increased metabolic need for ascorbate undoubtedly occurs in areas of the body not primarily involved in the disease and can be accounted for by such functions as the adrenals producing more adrenaline and corticoids; the immune system producing more antibodies, interferon (19, 20), and other substances to fight the infection; the macrophages utilizing more ascorbate with their increased activity; and the production and protection of c-AMP and c-GMP with the subsequent increased activity of other endocrine glands (21), etc. Also, there must be a tremendous draw on ascorbate locally by increased metabolic rates in the primarily infected tissues. The infecting organisms themselves liberate toxins which are neutralized by ascorbate, but in the process destroy ascorbate. The levels of ascorbate in the nose, throat, eustachian tubes, and bronchial tubes locally infected by a 100 gram cold must be very low indeed. With this acute induced scurvy localized in these areas, it is small wonder that healing can be delayed and complications such as chronic sinusitis, otitis media, and bronchitis, etc. develop. I had assumed that much of this ascorbate was used for functions somehow directly related to neutralizing the toxicity of viral and bacterial diseases. When ill, one has the internal sense that something of this nature is happening when bowel tolerance is approached. Recently, however, I had the personal experience of ingesting 48 grams in an hour and a half when I had a sudden hay fever reaction to roses. Upon withdrawal from the roses tolerance dropped rapidly to normal. This experience plus my experiences with many patients under emotional stress, would indicate that the adrenals are capable of utilizing large amounts of ascorbate with benefit if it is made available. This draw on ascorbate, from whatever source, lowers the blood level of ascorbate to a negligible level. I have coined the term ANASCORBEMIA for this condition. If this anascorbemia is not rapidly rectified by the oral administration of bowel tolerance doses of ascorbic acid or by intravenous administration of ascorbate, the remainder of the body is rapidly depleted of ascorbate and put at risk for disorders of the metabolic processes dependent upon vitamin C. The following problems should be expected with increased incidence with severe depletion of ascorbate: disorders of the immune system such as secondary infections, rheumatoid arthritis and other collagen diseases, allergic reactions to drugs, foods and other substances, chronic infections such as herpes, or sequelae of acute infections such as Guillain-Barre' and Reye's syndromes, rheumatic fever, or scarlet fever; disorders of the blood coagulation mechanisms such as hemorrhage, heart attacks, strokes, hemorrhoids, and other vascular thrombosis; failure to cope properly with stresses due to suppression of the adrenal functions such as phlebitis, other inflammatory disorders, asthma and other allergies; problems of disordered collagen formation such as impaired ability to heal, excessive scarring, bed sores, varicose veins, hernias, stretch marks, wrinkles, perhaps even wear of cartilage or degeneration of spinal discs; impaired function of the nervous system such as malaise, decreased pain tolerance, tendency to muscle spasms, even psychiatric disorders and senility; and cancer from the suppressed immune system and carcinogens not detoxified; etc. Note that I am not saying that ascorbate depletion is the only cause of these disorders, but I am pointing out that disorders of these systems would certainly predispose to these diseases and that these systems are known to be dependent upon ascorbate for their proper function. Not only is there the theoretical probability that these types of complications associated with infections or stresses could result from ascorbate depletion, but there was a conspicuous decrease in the expected occurrence of complications in the thousands of patients treated with oral tolerance doses or intravenous doses of ascorbate. This impression of marked decrease in these problems is shared by physicians experienced with the use of ascorbate such as Klenner (8, 9) and Kalokerinos (22). THE MISSING STRESS HORMONEStone (11) has described the genetic defect whereby the higher primates lost the ability to synthesize ascorbate. This defect is caused by a mutated defective gene for the liver enzyme, L-gulonolactone oxidase. The higher mammals (except for the higher primates) developed a feedback mechanism which increases ascorbate synthesis under the influence of external and internal stresses (23). There are many well-established functions of vitamin C that help in the handling of stress. When stressed, the higher mammals can augment these functions by this feedback mechanism. For the higher primates, including humans, ascorbate can amount to the MISSING STRESS HORMONE (4). I have seen strong clinical evidence that not only does the bowel tolerance to ascorbate increase under stress but that fully satisfying that potential use for ascorbate markedly reduces secondary diseases and complications following stress or primary disease. Since 1970, with teaching the bowel tolerance method of determining proper ascorbic acid doses to patients, I have not had to hospitalize a single patient for an acute viral disease or a complication from such a disease if the patient utilized the method. In some cases, such as with three cases of viral pneumonia, it was necessary to utilize intravenous ascorbate. Admittedly, I have been lucky because no patient has arrived with such severe symptoms as to necessitate immediate hospitalization. There have been many patients where there was no question that they would have required hospitalization in a very short period of time had not ascorbate been administered. Some patients not quite taking bowel tolerance doses, but taking significantly large doses of ascorbate, would not have as dramatic suppression of acute symptoms but would, nevertheless, avert complications. MONONUCLEOSISAcute mononucleosis is a good example because there is such an obvious difference between the course of the disease, with and without ascorbate. Also, it is possible to obtain laboratory diagnosis to verify that it is mononucleosis being treated. Early in this study a 23-year-old, 98-pound librarian with severe mononucleosis claimed to have taken 2 heaping tablespoons every 2 hours, consuming a full pound of ascorbic acid in 2 days. She felt mostly well in 3 to 4 days, although she had to continue about 20 to 30 grams a day for about 2 months. Many cases do not require maintenance doses for more than 2 to 3 weeks. The duration of need can be sensed by the patient. I had ski patrol patients back skiing on the slopes in a week. They were instructed to carry their boda bags full of ascorbic acid solution as they skied. The ascorbate kept the disease symptoms almost completely suppressed even if the basic infection had not completely resolved. The lymph nodes and spleen returned to normal rapidly and the profound malaise was relieved in a few days. It is emphasized that tolerance doses must be maintained until the patient senses he is completely well, or the symptoms will recur. HEPATITISAcute cases of infectious hepatitis have responded dramatically. Cases included two orthopaedic surgeons who probably acquired the disease pricking their hands at surgery and being inoculated with a patient's blood. With ascorbate treatment laboratory tests including the SGOT, SGPT, and bilirubins indicated rapid reversal of the disease. In one of these cases, with the doctorpatient and his treating physicians having difficulty believing that the ascorbate was responsible for the improvement, the ascorbate was discontinued. The condition of the patient rapidly deteriorated. The patient's wife took charge and doled out the ascorbate; again the disease rapidly subsided with laboratory findings returning to normal. Usually oral bowel tolerance doses will reverse hepatitis rapidly. Stools regularly return to normal color in 2 days. It generally takes about 6 days for the jaundice to clear, but the patient will feel almost well after 4 to 5 days. Because of the diarrhea caused by the disease, intravenous ascorbate may need to be used in very severe cases. Often large doses of ascorbic acid, taken orally despite diarrhea, will cause a paradoxical cessation of the diarrhea. Morishige has demonstrated the effectiveness of ascorbate in preventing hepatitis from blood transfusions (24). UNSICKThe phenomenon of symptoms returning repeatedly if the ascorbate is not continued in high doses is most convincing. It is possible to have symptoms come and go many times. In fact, there is often a feeling when titrating to bowel tolerance that symptoms are beginning to return just before taking the next dose. Often a patient will sense that he is probably catching some viral disease and that he is in need of large doses of ascorbic acid. If he is experienced in taking ascorbic acid he may be able to suppress more than 90% of the symptoms. He feels that he should take large amounts of ascorbate, does not feel quite right, and may have peculiar mild symptoms. I call this condition UNSICK. Recognition of this state is important because it can be mistaken for more serious conditions. INTRAVENOUS AND INTRAMUSCULAR ASCORBATESymptoms from acute viral diseases can most frequently be more permanently eliminated with intravenous sodium ascorbate. While it is true that tolerance doses of oral ascorbate will usually eliminate complications of acute viral diseases; at times, such as with certain cases of influenza, the large amount of oral ascorbate necessary to suppress symptoms over a period of a week or more, sometimes makes intravenous ascorbate desirable. Clinically large amounts of ascorbate used intravenously are virucidal (2, 5, 7, 8). The sodium ascorbate used intravenously and intramuscularly must contain no preservatives. Usually there is only a small amount of EDTA in the preparation to chelate trace amounts of copper and iron which might destroy the ascorbate. Solutions containing sodium ascorbate 250 or 500 mgm per cc can be obtained. The 250 mgm solutions may be used in young children intramuscularly in doses usually 350 mgm/kg body weight up to every 2 hours. When the volume of the material becomes too great for intramuscular injections, then the intravenous route should be used. Inadequate doses will be ineffective. Quite frequently a child initially refusing oral ascorbate will cooperate after injections if given the alternative. While this method of persuasion seems cruel, it is better than the complications which might otherwise occur. These intramuscular injections can be used in a crisis situation. Kalokerinos (22) describes cases where certain death in infants already in shock has been averted by emergency intramuscular ascorbate. For intravenous solutions concentrations of 60 grams per liter are made with the 250 or 500 mgm/cc sodium ascorbate diluted with Ringer's lactate, 1/2N saline, 1N saline, D5W, or distilled water for injection. I prefer the latter, but one has to be absolutely sure that an error is not made and pure water given. Ascorbate is more efficient intravenously than orally probably because chemical processes in the gut destroy a percentage of that orally administered. Doses of 400 to 700 mgm/kg of body weight per 24 hours usually suffice. Rate of infusion and the total amount administered can be determined by making sure that symptoms are suppressed and that the patient not become dehydrated or receive sodium too rapidly. Local soreness in the vein caused by too rapid infusion is relieved by slowing the intravenous infusion. One gram of calcium gluconate should be added to the bottles each day to prevent tetany. I have not yet seen a case of phlebitis develop as a result of ascorbate administration. This rarity of phlebitis possibly suggests that this condition sometimes has something to do with ascorbate depletion. Frequently I have the patient take oral doses of ascorbic acid at the same time he is taking intravenous sodium ascorbate. Bowel tolerance is actually increased by concomitant use of intravenous ascorbate. Care and experience is necessary with concomitant use because tolerance drops precipitously when the intravenous infusion is discontinued. BACTERIAL INFECTIONSAscorbic acid should be used with the appropriate antibiotic. The effect of ascorbic acid is synergistic with antibiotics and would appear to broaden the spectrum of antibiotics considerably. I found that penicillin-K orally or penicillin-G intramuscularly used in conjunction with bowel tolerance doses of ascorbic acid would usually treat infections caused by organisms ordinarily requiring ampicillin or other more modern synthetic penicillins. Cephalosporins were used in conjunction with ascorbic acid for staphylococcus infections. The combination of tetracycline and ascorbate was used for nonspecific urethritis; however, patients who had previously repeated recurrences of nonspecific urethritis found they were free of the disease with maintenance doses of ascorbate. I am not sure that the tetracycline was necessary even in the acute cases, but it was used for legal reasons. Some other cases of unknown etiology such as two cases of Reiter's disease and one case of acute anterior uveitis also responded dramatically to ascorbate. A most important point is that patients with bacterial infections would usually respond rapidly to ascorbic acid plus a basic antibiotic determined by initial clinical impressions. If cultures subsequently proved the selection of antibiotic incorrect, usually the patient was well by that time. In the case of a 45-year-old man who had developed osteomyelitis of the 5th metacarpal of the right hand following a cat bite, a partial amputation of the hand had been recommended and surgery scheduled. Consultants agreed. The patient delayed surgery and signed himself out of the hospital. He was given intravenous ascorbate 50 grams a day for 2 weeks. The infection resolved rapidly. While this patient had destruction of the distal end of the metacarpal, there has been no recurrence of the infection (25). This case illustrates the frequent problem of an indolent infection with an organism non-responsive to the most sophisticated antibiotic treatment which then may respond rapidly to treatment with intravenous ascorbate. Treating simultaneously with the appropriate antibiotic plus ascorbate has the additional advantage that if, unexpectedly, the infection is actually viral, the infection will be suppressed and the incidence of allergic reaction to the antibiotic reduced. VITAMIN C AND ALLERGYPatients seemed not to develop their first allergic reaction to penicillin when they had taken bowel tolerance ascorbate for several doses. Among the several thousand patients given penicillin, two cases of brief rash were seen in patients who had taken their first dose of penicillin along with their first dose of ascorbate. If one understands the reasons for bowel tolerance doses of ascorbate, it is obvious that these patients were not as yet "saturated." I saw three patients who had taken penicillin without ascorbate who had developed an urticarial rash. These cases rapidly responded to oral ascorbic acid. Only a single dose of antihistamine was usually used. I would have anticipated longer reactions in most of these cases. I saw one case of a delayed serum sickness type of penicillin reaction in a ten-year-old girl who had not taken ascorbate previously. The rash in this patient did not immediately respond to ascorbic acid. The rash took about two weeks to completely resolve; however, if the ascorbate was not taken regularly to tolerance, the rash would worsen. It was difficult to maintain high doses in this patient. Patients who had known-previous-allergic reactions to penicillin were never given the antibiotic anticipating that vitamin C would protect them. I suspect that the deficit of body ascorbate produced by disease may have something to do with malfunction of the immune system and the development of allergies. However, whether ascorbate may give some protection from an antibiotic known previously to cause an allergic reaction in a patient, when subsequent reactions might involve anaphylaxis, is a question which must be approached very carefully. Certainly, inadequate doses of ascorbate could be disastrous. Patients with mononucleosis, untreated with ascorbate, have a very high incidence of allergic reaction to penicillin. It is interesting that this same disease seems to cause some of the highest bowel tolerances of any disease. As can be seen from the previous discussion of the increasing bowel tolerance phenomenon, there is undoubtedly increased utilization of ascorbate under stressful conditions. If this increased utilization creates a deficit, there may be malfunctions of various systems of the body such as the immune system which are dependent on ascorbate. Therefore, it should not be surprising that certain malfunctions of the immune system and adrenal glands associated with stress might be ameliorated by ascorbate. Hay fever is controlled in the majority of patients. Bowel tolerance doses are usually required only at the peak of the season; otherwise, more modest doses suffice. Many patients find the effect of ascorbate more satisfactory than immunizations or antihistamines and decongestants. The dosages required are frequently proportional to exposure to the antigen. Asthma is most often relieved by bowel tolerance doses of ascorbate. A child regularly having asthmatic attacks following exercise is usually relieved of these attacks by large doses of ascorbate. So far all of my patients having asthmatic attacks associated with the onset of viral diseases have been ameliorated by this treatment. Large clinical studies will be necessary to prove this point, but for now prudent practice would be to take large doses ofascorbate when stressed or when ill. This theory begins to make some sense of the observation that many patients will develop allergic disorders or other diseases following combinations of stress, disease, and malnutrition. Immunologists should be particularly interested in the control of these allergic problems and particularly the dramatic responses of cases of ankylosing spondylitis, Reiter's disease, and acute anterior uveitis. All three of these problems have a high association with the HLA-B27 antigen. The possibility that ascorbate might have some value in controlling the immune response at the gene level should be thoroughly investigated because there could be some basic implications in histocompatibility (graft acceptance), cancer control, and destruction of foreign invaders. Ascorbate would appear to help stabilize some homeostatic mechanisms. CANDIDA ALBICANSYeast infections occur less frequently in patients treated with antibiotics if bowel tolerance doses of ascorbic acid are simul- taneously used. Ascorbic acid seems to reduce the systemic toxicity considerably but does not eliminate the primary infection. It has been helpful to patients with allergic problems secondary to candida. FUNGUS INFECTIONSAlthough ascorbic acid should be given in some form to all sick patients to help meet the stress of disease, it is my experience that ascorbate has little effect on the primary fungal infections. Systemic toxicity and complications can be reduced in incidence. It may be found that appropriate antifungal agents will better penetrate tissues saturated in ascorbate. TRAUMA, SURGERY, AND BURNSSwelling and pain from trauma, surgery, and burns are markedly reduced by bowel tolerance doses of ascorbic acid. Doses should be given a minimum of 6 times a day for trauma and surgery. Burns can require hourly doses. Serious burns, major trauma, and surgery should be treated with intravenous ascorbate. The effect of ascorbate on anesthetics should be studied. Barbiturates and many narcotics are blocked, (26) so their use as anesthetic agents will be limited when ascorbate is used during surgery. While practicing orthopaedic surgery, I had some experience with trauma cases in which I used ascorbic acid post-operatively. There was virtual elimination of confusion in elderly patients following major surgeries such as with hip fractures when ascorbate was given. This confusion is commonly ascribed to fat embolization and the subsequent inflammation provoked in the tissues by the emboli. I did several menisectomies where one knee had been done before vitamin C was used, and the other side after vitamin C was used. The pain and post-operative recovery time were lessened considerably. The amount of inflammation and edema following injury and surgery were markedly reduced. The pain medications used were relatively minimal. My limited experience in replacing skin flaps avulsed by trauma indicated a whole degree of lessened difficulties with much greater success. Anyone who has done animal surgery other than on humans is impressed by the rapid recovery rate. Humans loaded with ascorbate would appear to recover similarly to the animals which make their own ascorbate in response to stress. In the past, vitamin C administered to patients in hospitals post-operatively has been in trivial amounts never exceeding several grams. I predict that reimplantations of major amputations, even transplant surgeries, and especially fine surgeries of the eyes, ears, or fingers will enjoy a phenomenal increase in success rate when ascorbate is utilized in doses of 100 grams or more per 24 hours. The limited stress-coping mechanisms of humans seems to be the result of rapid ascorbate depletion. With surgery this leads to vascular thrombosis, hemorrhage, infection, edema, drug reactions, shock, adrenal collapse with limited adrenaline and steroid production, etc. CANCERI have avoided the treatment of cancer patients for legal reasons; however, I have given nutritional consults to a number of cancer patients and have observed an increased bowel tolerance to ascorbic acid. Were I treating cancer patients, I would not limit their ascorbic acid ingestion to a set amount but would titrate them to bowel tolerance. Ewan Cameron's advice against giving cancer patients with widespread metastasis large amounts of ascorbate too rapidly at first should be heeded. He found that sometimes extensive necrosis or hemorrhage in the cancer could kill a patient with widespread metastasis if the vitamin was started too rapidly (16). Hopefully, in the future ascorbic acid will be among the initial treatments given cancer patients. The additional nutritional needs of cancer patients are not limited to ascorbic acid, but certainly the stress involved with having the disease depletes ascorbate levels in the body. Ascorbate should be used in cancer patients to avert disorders of ascorbate deficiency in various systems of the body including the immune system. BACK PAIN FROM DISC DISEASEGreenwood (27) observed that 1 gram a day would reduce the incidence of necessary surgery on discs. At bowel tolerance levels, ascorbic acid reduces pain about 50% and lessens the difficulties with narcotics and muscle relaxants (2). It is not, however, the only nutritional support that patients with back pain should receive. ARTHRITISBowel tolerance is not increased by degenerative arthritis although occasionally ascorbate has some beneficial effect. Ankylosing spondylitis and rheumatoid arthritis do increase tolerance. Clinical response varies. Norman Cousins (28) curing his own ankylosing spondylitis with ascorbate is not unexpected. With these and other collagen diseases, food and chemical allergies can sometimes be found. It may be that the blocking of allergic reactions with augmented adrenal function is one of the reasons these patients are sometimes benefitted. SCARLET FEVERThree cases with typical sandpaper-like rash, peeling skin, and diagnostic laboratory findings of scarlet fever have responded within an hour or overnight. I think this immediate response is due to the neutralization of the small amount of streptococcus toxin responsible for the disease. Although I have not seen a case of acute rheumatic fever, I would anticipate rapid effects. HERPES: COLD SORES, GENITAL LESIONS, AND SHINGLESAcute herpes infections are usually ameliorated with bowel tolerance doses of ascorbic acid. However, recurrences are common especially if the disease has already become chronic. Zinc in combination with ascorbic acid is more effective for herpes; however, caution and regular monitoring of patients on zinc should be done. For chronic herpes, intravenous ascorbate may also be of benefit. CRIB DEATHS (SUDDEN INFANT DEATH SYNDROME)I would agree with Kalokerinos (22) and Klenner (8) that crib deaths are often caused by sudden ascorbate depletions. The induced scurvy in some vital regulatory center kills the child. This induced deficiency is more likely to occur when the diet is poor in vitamin C. All of the epidemiologic factors predisposing to crib deaths are associated with low vitamin C intake or high vitamin C destruction. MAINTENANCE DOSESMaintenance doses are established by the patient taking bowel tolerance doses 6 times a day for at least a week. He observes if there is any unexpected benefit such as clearing of sinuses, decrease in allergies, increase in energy, etc. Should any chronic problem be benefitted, then the dose is decreased to the minimum amount producing the effect. Otherwise a dose such as 4 to 10 grams a day divided in 3 to 4 doses is recommended. In addition, the patient is told to increase the dose on stressful days. If a patient well tolerates ascorbic acid dissolved in water, then after a short period of time his taste will begin to regulate the dosages. Most patients can easily sense their ascorbate needs. Patients who take ascorbate in large amounts over a long period of time should probably suppliment with vitamin A and a multiple mineral preparation. The "Fortified Formulation for Nutritional Insurance" of Roger Williams (29) is recommended as a base. COMPLICATIONSIt is my experience that ascorbic acid probably prevents most kidney stones. I have had a few patients who had had kidney stones before starting bowel tolerance doses who have subsequently had no more difficulty with them. Acute and chronic urinary tract infections are often eliminated; this fact may remove one of the causes of kidney stones. Six patients have had mild pain on urination; five of these patients were over fifty and none had stones. Three out of thousands had a light rash which cleared with subsequent doses. It was difficult to evaluate the cause of this because of concomitant infections. Several patients had discoloration of the skin under jewelry of certain metals. A few patients complaining of small sores in the mouth with the taking of small doses of ascorbate had them clear with bowel tolerance doses. Patients with hidden peptic ulcers may have pain, but some are benefitted. Mineral ascorbates can be used for maintenance doses in these cases. Two patients who had mild epigastric discomfort with maintenance doses of ascorbic acid who after being given ascorbate by vein for several days were then able to tolerate the acid orally. It is my experience that high maintenance doses reduce the incidence of gouty arthritis. I have not seen difficulties with giving large amounts of ascorbic acid to patients with gout. Almost all my patients have been Caucasian, so I have no comment on the report that ascorbate can cause certain blood problems in certain non-white groups (30). There has been no clinical evidence as Herbert and Jacob (31) suspected that ascorbic acid destroys vitamin B12. If maintenance doses of ascorbic acid in solution are used over very long periods of time I would rinse the teeth after each dose. I would not brush my teeth with calcium ascorbate. There is a certain dependency on ascorbic acid that a patient acquires over a long period of time when he takes large maintenance doses. Apparently, certain metabolic reactions are facilitated by large amounts of ascorbate and if the substance is suddenly withdrawn, certain problems result such as a cold, return of allergy, fatigue, etc. Mostly, these problems are a return of problems the patient had before taking the ascorbic acid. Patients have by this time become so adjusted to feeling better that they refuse to go without ascorbic acid. Patients do not seem to acquire this dependency in the short time they take doses to bowel tolerance to treat an acute disease. Maintenance doses of 4 grams per day do not seem to create a noticeable dependency. The majority of patients who take over 10-15 grams of ascorbic acid per day probably have certain metabolic needs for ascorbate which exceed the universal human species need. Patients with chronic allergies often take large maintenance doses. The major problem feared by patients benefiting from these large maintenance doses of ascorbic acid is that they may be forced into a position where their body is deprived of ascorbate during a period of great stress such as emergency hospitalization. Physicians should recognize the consequences of suddenly withdrawing ascorbate under these circumstances and be prepared to meet these increased metabolic needs for ascorbate in even an unconscious patient. These consequences of ascorbate depletion which may include shock, heart attack, phlebitis, pneumonia, allergic reactions, increased susceptibility to infection, etc., may be averted only by ascorbate. Patients unable to take large oral doses should be given intravenous ascorbate. All hospitals should have supplies of large amounts of ascorbate for intravenous use to meet this need. The millions of people taking ascorbic acid makes this an urgent priority. Patients should carry warnings of these needs in a card prominently displayed in their wallets or have a Medic Alert type bracelet engraved with this warning. CONCLUSIONThe method of titrating a patient's dosage of ascorbic acid between the relief of most symptoms and bowel tolerance has been described. Either this titration method or large intravenous doses are absolutely necessary to obtain excellent results. Studies of lesser amounts are almost useless. The oral method cannot by its very nature be investigated by double blind studies because no placebo will mimic this bowel tolerance phenomenon. The method produces such spectacular effects in all patients capable of tolerating these doses, especially in the cases of acute self-limiting viral diseases, as to be undeniable. A placebo could not possibly work so reliably, even in infants and children, and have such a profound effect on critically ill patients. Belfield (32) has had similar results in veterinary medicine curing distemper and kennel fever in dogs with intravenous ascorbate. Although dogs produce their own ascorbate, they do not produce enough to neutralize the toxicity of these diseases. This effect in animals could hardly be a placebo. It would be possible to conduct a double blind study on intravenous ascorbate; however, doses would have to be determined by someone experienced with this method. Part of the difficulty many have with understanding ascorbate is that claims for its benefits seem too many. Most of these clinical results merely indicate that large doses of ascorbate augment the healing abilities of the body already known to be dependent upon minimal doses of ascorbate. I anticipate that other essential nutrients will be found being utilized at unsuspectedly rapid rates in disease states. Compli- cations caused by failures in systems dependent upon those nutrients will be found. The magnitude of supplimentations necessary to avert those complications will seem extraordinary by standards accepted today. REFERENCES1. Cathcart, R.F. Clinical trial of vitamin C. Medical Tribune, June 25, 1975. 2. Cathcart, R.F. Clinical use of large doses of ascorbic acid. Presented at the annual meeting of the California Orthomolecular Medical Society, San Francisco, February 19, 1976. 3. Cathcart, R.F. Vitamin C as a detoxifying agent. Presented at the annual meeting of the Orthomolecular Medical Society, San Francisco, January 21, 1978. 4. Cathcart, R.F. Vitamin C - The missing stress hormone. Presented at the annual meeting of the Orthomolecular Medical Society, San Francisco, March 3, 1979. 5. Cathcart, R.F. The method of determining proper doses of vitamin C for the treatment of disease by titrating to bowel tolerance. J. Orthomolecular Psychiatry, 10:125-132, 1981. 6. Klenner, F.R. Virus pneumonia and its treatment with vitamin C. J. South. Med. and Surg., 110:60-63, 1948. 7. Klenner, F.R. The treatment of poliomyelitis and other viral diseases with vitamin C. J. South. Med. and Surg., 111:210-214, 1949. 8. Klenner, F.R. Observations on the dose and administration of ascorbic acid when employed beyond the range of a vitamin in human pathology. J. App. Nutr., 23:61-88, 1971. 9. Klenner, F.R. Significance of high daily intake of ascorbic acid in preventive medicine. J. Int. Acad. Prev. Med., 1:45-49, 1974. 10. Stone, I. Studies of a mammalian enzyme system for producing evolutionary evidence on man. Am. J. Phys. Anthro., 23:83-86, 1965. 11. Stone, I. Hypoascorbemia: The genetic disease causing the human requirement for exogenous ascorbic acid. Perspectives in Biology and Medicine, 10:133-134, 1966. 12. Stone, I. The Healing Factor: Vitamin C Against Disease. Grosset and Dunlap, New York, 1972. 13. Pauling, L. Vitamin C and the Common Cold. W.H. Freeman and Company, San Francisco, 1970. 14. Pauling, L. Vitamin C, the Common Cold, and the Flu. W.H. Freeman and Company, San Francisco, 1976. 15. Cameron, E. and Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA, 73:3685-3689, 1976. 16. Cameron, E. and Pauling, L. The orthomolecular treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA, 75:4538-4542, 1978. 17. Cameron, E. and Pauling, L. Cancer and Vitamin C. The Linus Pauling Institute for Science and Medicine, Menlo Park, 1979. 18. Williams, R.J. Biochemical Individuality. John Wiley, New York, 1956. University of Texas Press, Austin, Texas, 1973. 19. Siegel, B.V. Enhancement of Interferon Response by poly(rI).- poly(rC) in Mouse Cultures by Ascorbic Acid. Nature 254:531-532, 1975. 20. Siegel, B.V., Morton, J.I. Vitamin C and the Immune Response. Experientia 33:393-395, 1977. 21. Lewin, S. Vitamin C: Its Molecular Biology and Medical Potential. Academic Press, London, 1976. 22. Kalokerinos, A. Every Second Child, Thomas Nelson, Australia, 1974. 23. Subramanian, N. et al. Detoxification of histamine with ascorbic acid. Biochemical Pharmacology. 27:1671-1673, 1973. 24. Murata, A. Virucidal activity of vitamin C: Vitamin C for the prevention and treatment of viral diseases. Proceedings of the First Intersectional Congress of Microbiological Societies, Science Council of Japan, 3:432-442, 1975. 25. Salaman, M. Fighting infection-the cat and the "C". Let's Live, 128-130, April 1980. 26. Libby, A.F. and Stone, I. The hypoascorbemia-kwashiorkor approach to drug addiction therapy: A pilot study. J. Orthomolecular Psychiatry, 6:300-308, 1977. 27. Greenwood, J. Optimum vitamin C intake as a factor in the preservation of disc integrity. Medical Annals of the District of Columbia, 33:274-276, 1964. 28. Cousins, N. Anatomy of an Illness as Perceived by the Patient. W.W. Norton & Company, New York, 1979. 29. Williams, R.J. The Prevention of Alcoholism Through Nutrition. Bantam Books, New York, 1981. 30. Campbell, G.D. Jr., Steinberg, M.H. and Bower, J.D. Ascorbic acid induced hemolysis in G-6-PD deficiency. Ann. Int. Med. 82:810, 1975. 31. Herbert, V. and Jacob, E. Destruction of vitamin B12 by ascorbic acid. JAMA, 230:241-242, 1974. 32. Belfield, W.O. and Stone, I. Megascorbic prophylaxis and megascorbic therapy: A new orthomolecular modality in veterinary medicine. Journal of the International Academy of Preventive Medicine, 2:10-26, 1975.
Robert Cathcart, M.D.650-949-2822Copyright (C), 1994 and prior years, Robert F. Cathcart, M.D. Permission granted to distribute via the internet as long as material is distributed in its entirity and not modified. In Extremis: First Aid for Advanced Cancer
Counter indications to this approach are few. However they include anyone with kidney failure, or on dialysis, or with uncommon forms of iron overload. Responsible physicians should also screen for red blood cell glucose-6 phosphate dehydrogenase deficiency, a rare condition whose presence can lead to haemolytic crisis involving red blood cell breakdown. The very large doses should also be built up to gradually over some days to establish good tolerance, starting at 15 grams for 1 or 2 sessions, then to 50 grams and, if necessary, to 100 grams. The exact dose is determined by the individual's plasma saturation by Vitamin C immediately after an infusion. WARNING: To avoid the well-documented Rebound Effect, which can lead to scurvy, this treatment should not be stopped abruptly. Patients should be gradually weaned off it over a period of weeks, or even months, and oral vitamin C therapy should continue indefinitely and on the days in between the IVC infusions. The American Dr Hugh Riordan M.D. is probably the world expert on this approach. His institute, The Center for the Improvement of Human Functioning, has just completed a 10 year research project on high dose intravenous C and cancer, and his patented method recently underwent Phase I clinical trials at the University of Nebraska medical school hospital. These trials have established the non-toxicity of this treatment for cancer, and Dr Riordan is now proceeding with a Phase II clinical trial, under the auspices of the National Institutes of Health, using therapeutic doses of vitamin C on Renal Adenoma patients. Dr Riordan has also published several successful case histories, including the results of treatment on a late-stage lung cancer patient - now cancer free several years on -, in The Journal of Orthomolecular Medicine. I would recommend anyone interested in this to get in touch with Dr Riordan, and to consult him generally for nutritional strategies against cancer, in particular as Dr Riordan has at his disposal some of the most refined lab-tests in the world for determining individual bio-chemical profiles and needs in cancer, (or indeed any other condition). These tests should be a standard in determining optimal individual nutritional therapy. Unfortunately, as yet, they are not widely available. Dr Riordan is also recruiting "end-stage" cancer patients for a trial of a new immunotherapy for cancer, in which the immune system is taught to recognise and destroy the cancer cells in its midst that it usually overlooks. The trials are free. But you must be able to travel to the U.S. However, there are a number of other intravenous vitamin C practitioners throughout the world. The International Society for Orthomolecular Medicine, (Ms Claire D'Intino) can give you the name and address of your nearest orthomolecular physician worldwide. (Or see the Countries List in the Resource Section.) The Doctors and Organisations pages list a few English speaking practitioners, all M.D.s, who also offer excellent alternative and complementary, immunotherapeutic approaches to cancer. For maximum efficacy, they should follow Dr Riordan's treatment protocol, available here, and on request from the Center for the Improvement of Human Functioning: DR HUGH RIORDAN Center For The Improvement Of Human Functioning
Ascorbic Acid Orally - Not for IV However, Klenner and I have used sodium ascorbate intravenously and when used rarely intramuscularly. This is confused by the fact that even Klenner referred to the intravenous solutions as ascorbic acid. However, I had talked with him before his death and also talked with his wife and nurse, Annie Klenner, and they said it was the sodium ascorbate powder that he simply mixed in water that he used. See http://www.orthomed.com/civprep.htm All this is further confused by the fact that the commonly available form used by many orthomolecular physicians is from Merit Pharmaceuticals and it is labeled ascorbic acid. It is, however, made as prescribed in the U.S. Pharmacopoeia made from ascorbic acid and then buffered to a pH of 5 or 6 (I have to check for that exact pH specified) with sodium hydroxide or sodium bicarbonate. This, in effect, is actually mainly sodium ascorbate. It cannot be pure ascorbic acid because that would be a pH of 3.5 and that would be far too acid. The problem here is that everyone keeps talking about ascorbic acid intravenously when they really should be using sodium ascorbate or at least highly buffered ascorbic acid. All this is bound to be causing mistakes. Last week I was consulting with three doctors at the Mayo Clinic that are allowing an old patient of mine I had seen years ago, die of pneumonia rather than give her sodium ascorbate. She was in the habit of taking 30 grams of ascorbic acid by mouth per day. I had cured her of transverse myelitis years ago to the astonishment of the transverse myelitis experts and the University of California Medical Center in San Francisco with several weeks of sodium ascorbate intravenously. They, of course, did nothing to follow up on this cure with further investigations. Anyway, the Mayo Clinic doctors were giving the usual nonsense arguments about not using C like that it would cause metabolic acidosis. I pointed out how ridiculous this was to worry about metabolic acidosis when you were giving an alkali solution. But they kept talking about ascorbic acid. There is in the literature a story about a black man who was given intravenous ascorbic acid for a small burn who died of subsequent kidney failure. Here again we have the ascorbic acid story. In some foreign countries, they are beginning to pick up on this intravenous vitamin C for cancer. I want to make sure they use either sodium ascorbate or highly buffered ascorbic acid. There are bound to be mistakes if we keep talking about ascorbic acid intravenously without qualification. So please modify all the references to ascorbic acid intravenously to preferably sodium ascorbate or at least highly buffered ascorbic acid. Also it is important that it not have preservatives. Thanks. Dr. Robert Cathcart, III, MD www.orthomed.com
Intravenous
Vitamin Therapy
The benefits of long-term vitamin C consumption in excess of the U.S. government recommended daily allowance (RDA) are widely acknowledged and include reduced risks of cancer, cardiovascular disease, and cataracts. Higher than RDA vitamin C intakes have been associated with increases in HDL cholesterol, decreases in LDL cholesterol oxidation, decreased blood pressure, and decreased cardiovascular mortality. Vitamin C enhances non-heme iron absorption in individuals with low iron status. Helpfulness has been reported for severe attacks of ulcerative colitis, advanced human cancer, and reticulum cell sarcoma. Doses in these reports ranged from 50 to 150 gm intravenous per day, with no adverse effects reported. The manufacturers' literature states that doses as high as 6 gm per day has been administered without toxicity. High dose IV vitamin C has been used to treat optic neuritis, and was found to produce equivalent results to oral or intravenous corticosterone, or oral vitamin B12. No adverse events were encountered among 25 patients treated. The possibility of its working through its action on free radicals was raised. Higher dose vitamin C (170 mg/kg/24 hours) was shown to have no adverse effects upon guinea pigs. These animals had 70% body surface area deep thermal burns. Animals receiving the vitamin C had lower water content of the burned skin, suggesting that postburn capillary permeability was minimized by the use of vitamin C. With even higher doses, the authors were able to reduce the 24 hour resuscitation fluid volume from 4 ml/kg/% burn to 1 ml/kg/% burn, while still maintaining adequate cardiac output. In contrast, with a lower dosage and measured only 5 hours after the injury, vitamin C infusions had no effect on graded scald produced burns, at least in terms of changes in microvascular permeability or in edema formation among dogs with hind-paw lesions. Pre-burn infusions did significantly attenuate burn-induced increases in paw weight gain, and no adverse reactions were encountered. Oxidized LDL cholesterol (oxLDL) induced leukocyte adhesion to both microvascular and macrovascular (aortic wall) endothelium, can be prevented by pre-treatment of hamsters with oral or intravenous vitamin C. The mechanism of action was thought to be the scavenging of reactive oxygen species. Vitamin E and probucol did not show these effects. Vitamin C Therapy (Studies): A 42 year old male with widely disseminated, biopsy-proven reticulum cell sarcoma was treated with high dose (100mg infusion Vitamin C. He experienced two complete, spontaneous regressions of his illness coinciding exactly in time with, intravenous vitamin C administration. He remains alive 17 years later. Of course, placebo has been reported to accomplish as much (the drug krebazolin), but that patient died after his second spontaneous regression of cancer when he learned the the drug in which he so believed had been declared by the FDA to be worthless. Vitamin C Therapy (Products): Vitamin C Therapy (Stability Issues): Trissel has reviewed available studies on physical compatibility of vitamin C with other products for injection. Vitamin C is compatible with 6% Dextran in 5% dextrose or 0.9% sodium chloride, dextrose-Ringer's solution combinations included those that are lactated, dextrose-saline combinations, 2.5% dextrose in water, 5% dextrose in half normal saline, 5 or 10% dextrose in water, 10% fat emulsion solution (for 48 hours), 10% fructose in normal saline or water, 5 or 10% invert sugar in normal saline or water, Ionosol products, lactated or non-lactated Ringer's injection solutions, Half-normal or normal saline, 1/6th molar sodium lactate, amikacin sulfate in all solutions, calcium chloride, calcium gluceptate, calcium gluconate, cephalothin sodium, chloramphenicol solution, chlorpromazine HCl, colistimethate sodium, cyanocobalamin (no loss of activity for either at 24 hours when protected from light), diphenhydramine HCl, heparin sodium, kanamycin sulfate, methicillin sodium, methyldopate HCl, penicillin G potassium, polymyxin B sulfate, prednisolone sodium phosphate, procaine HCl, prochlorperazine edisylate, promethazine HCl, and verapamil HCl. Conflicting results were found for aminophylline (higher doses were incompatible) and erythromycin lactobionate. Physical incompatibility was found for nafcillin sodium, sodium bicarbonate solution. Loss of all bleomycin activity occurred after 1 week together in solution. Precipitation occurred after several hours in solution with warfarin sodium. Precipitation occurred after 24 hours for etomidate, 7 days for propofol, and 24 hours for thiopental sodium. More importantly, TPN solution number 189 was studied with vitamin C. No incompatibility was found up to 24 hours (not studied afterwards). TPN # 189 consists of 500 ml of 10% amino acids with electrolytes (Synthamin 17 with electrolytes), 500 ml of 50% Dextrose, 2.2 mM calcium, 2.5 mM magnesium, 42.5 mM potassium, 45 mM sodium, 15 mM phosphorous, 55.65 mM chloride, 81.25 mM acetate, and 1 ml trace mineral solution. Other studies of vitamin C in parenteral nutrition solutions have shown no loss of activity so long as the mixture is protected from light. A 35% loss of activity was seen at 39 hours when the mixture was exposed to light continually. Thirty to forty percent of vitamin C activity was lost after 24 hours when ascorbic acid was added to parenteral nutrition solutions consisting of amino acids, dextrose, electrolytes, multivitamins, and trace minerals in 3 liter PVC bags stored at 3 to 7 degrees C. The degradation slowed as the oxygen supply was reduced to the diffusion through the bag. About a 55 to 65% loss of activity was reported after 7 days of storage. The oxidation of the ascorbic acid was catalyzed by metal ions, especially copper. In the absence of copper, less than 10% loss of activity occurred at 24 hours. No immediate incompatibility or precipitation was observed over the 7 days. Extensive decomposition of ascorbic acid and folic acid was reported in a parenteral nutrition solution composed of amino acids 3.3%, dextrose 12.5%, electrolytes, trace elements, and multi-vitamin infusion -12 (USV) in PVC bags. Half-lives were 1.1, 2.9, and 8.9 hours for ascorbic acid and 2.7, 5.4, and 24 hours for folic acid stored at 24 degrees C. in daylight, 24 degrees C. in darkness, and 4 degrees C. in darkness, respectively. Catalyzing metal ions increased the rate of decomposition greatly. Interactions with other vitamins present increased the rate of decomposition. In another parenteral nutrition solution composed of amino acids (Kabi-Vitrum), dextrose 30%, and fat emulsion 20% (Kabi-Vitrum) in a 2:1:1 ratio with electrolytes, trace elements, and both fat- and water-soluble vitamins, no significant loss of activity of retinyl palmitate, alpha tocopherol, thiamine mononitrate, sodium riboflavin-5'-phosphate, pyridoxine HCl, nicotinamide, folic acid, biotin, sodium pantothenate, and cyanocobalamin were found at 96 hours when the solution was stored in darkness at 2 to 8 degrees C. Sodium ascorbate and its biologically active degradation product, dehydroascorbate, totaled 59 and 42% of the starting concentration at 24 and 96 hours, respectively. No precipitation or incompatibilities were found. No significant loss of activity was found in a simulated infusion over 24 hours at room temperature without light protection. This procedure did increase the decomposition rate of vitamin C, however, with ascorbate showing 51% concentration and dehyrdroascorbate showing 65% concentration. Light protection did not significantly change this degradation rate at room temperature. HPLC analysis of the stability of vitamin C in parenteral nutrition solutions with and without fat emulsions showed retention of 90% vitamin C content for 12 hours when the solutions were exposed to fluorescent light and for 24 hours when they were protected from light. Storage in a cool, dark place resulted in retention of activity for 7 days. Ascorbic acid loss was studied with MVI-12 (Armour) admixed in parenteral nutrition solutions containing different amino acid products, with or without Intralipid 10%, and stored in either glass bottles or PVC bags, either refrigerated or at room temperature. Ascorbic acid was lost under all conditions at room temperature and not at refrigerated temperature. Losses were greater in PVC bags than glass bottles. No immediate incompatibilities or delayed precipitations were found. One can conclude from all these studies that some loss of vitamin C activity is inevitable and that it is prevented by light protection, preparing mixtures for infusion as close as possible to the time of infusion, and by giving higher concentrations of vitamin C than needed, to counteract degradation. Since no physical incompatibilities were found, giving higher concentrations seems logical, especially when trace minerals are found in the solution. The higher the mineral content of the solution and the longer from time of preparation to administration, probably the higher the vitamin C content should be. Further studies are needed in clinical settings to determine the extent of degradation expected and the amounts of vitamin C which should be given (at different mineral concentrations) to achieve desired dosing. Intravenous Infusions: Studies of Multiple
Nutrients: Intravenous B Vitamin Infusion: Thiamine. Three patients who developed Wernicke’s encephalopathy despite being given intravenous thiamine have been reported. These authors suggested that the amount of intravenous thiamine commonly given is much too low. Therefore, we propose that the PT Committee allow us to increase the amount of Vitamin C given to the 2 gm to 25 gm range, depending upon the patient. -------------------------------------------------------------------------------- References: Bendich A, Langseth L. The Health Effects
of Vitamin C Supplementation: A Review. J Am Coll Nutr 1995;
14(2): 124-36. I.V. VITAMIN C Recent studies demonstrate that vitamin C, infused intravenously, can achieve a plasma concentration high enough to exert cytotoxic effects on many types of cancer cells. Interestingly, vitamin C has both anti-oxidant and oxidant activities. At low to moderate dosage (achieved by taking Vitamin C orally), vitamin C is a potent antioxidant with important functions in immune response, wound healing and recycling of other antioxidants such as vitamin E. However at large dosage (achieved by taking Vitamin C intravenously), vitamin C can induce oxidative damages to cancer cell DNA, mainly through the increased production of hydrogen peroxides. This increase in oxidative stress preferentially induces cancer cells to undergo apoptosis (i.e. cell death) since cancer cells are relatively deficient in catalase, an enzyme that neutralizes hydrogen peroxide. Studies completed in Japan have successfully demonstrated that vitamin C infusion can enhance the therapeutic effects of chemotherapies. I.V. vitamin C is usually given 3 times per week for at least 4 weeks, according to the following schedule:
Note: This schedule is typical for a patient not currently on chemotherapy or radiation. Schedule and dosage may vary depending on clinical symptoms, lab and/or conventional treatment schedules. SIDE EFFECTS/COMPLICATIONS: A phase I study done
at the University of Nebraska medical centre has shown no
side effects or toxicities associated with intravenous vitamin
C procedure. However, some relative contra-indications and
potential side effects to be considered are:
|
||||||||||||||||||||||||||